[ad_1]
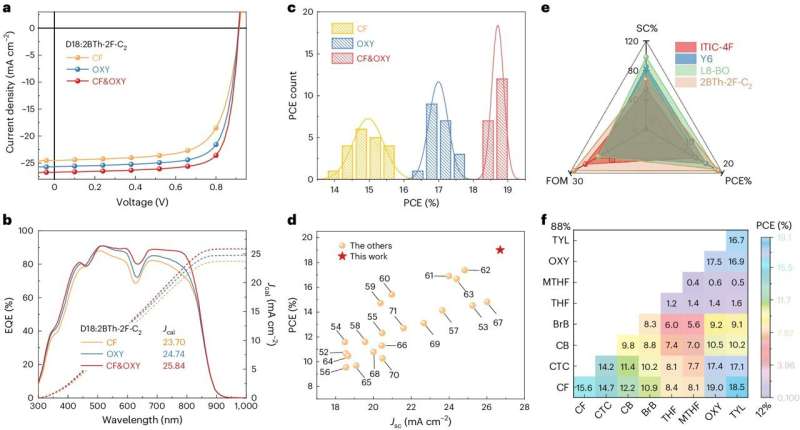
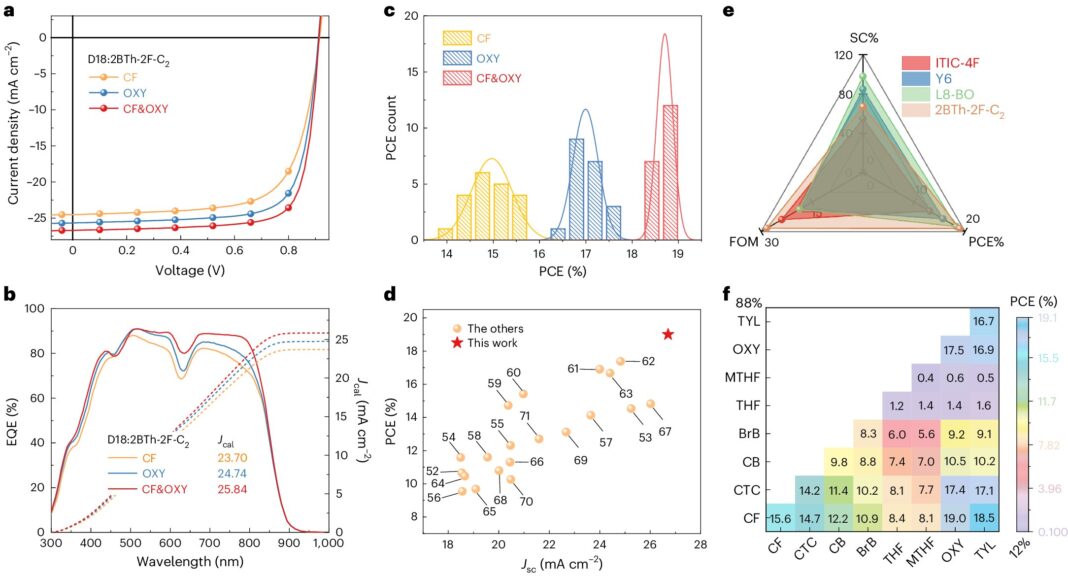
Device performances. Credit: Natural Energy (2024). DOI: 10.1038/s41560-024-01564-0
The power-conversion efficiencies (PCEs) of natural photo voltaic cells based mostly on compounds generally known as polymer donors and fused ring electron acceptors (FREAs) have just lately exceeded 19%. In distinction, natural photo voltaic cells based mostly on non-fused ring electron acceptors (NFREAs), cheaper compounds characterised by non-fused (i.e., separate) fragrant rings, have to date proven disappointing effectivity. by about 16%.
As synthesizing NFREAs is less expensive than synthesizing FREAs, creating extra environment friendly photo voltaic cells based mostly on these supplies might have necessary implications. Specifically, it might facilitate the widespread adoption of natural photo voltaic cells, thus probably contributing to decreasing emissions and mitigating environmental points.
Researchers at Shanghai Jiao Tong University, Qingdao University and different institutes in China have just lately proposed a brand new technique to make extra environment friendly natural photo voltaic cells based mostly on NFRAs. This technique, outlined in a paper printed in Natural Energydepends on the usage of a solvent based mostly on chloroform (CF) and o-xylene (OXY), in addition to a solid-state additive that additional enhances the crystallization of NFRAs, thus enabling the next PCE in photo voltaic cells based mostly on these compounds.
“Non-fused ring electron acceptors (NFREAs) probably have decrease artificial value than their fused counterparts,” Rui Zeng, Ming Zhang and their colleagues wrote of their paper. “However, the low spine planarity and the presence of enormous substituents harm the crystallinity of NFREAs, disrupting cost transport and the formation of bicontinuous morphology in natural photo voltaic cells. We present that the binary solvent system could be individually can management the crystallization and section separation of the donor polymer (eg, D18) and the NFREA (eg, 2BTh-2F-C2).”
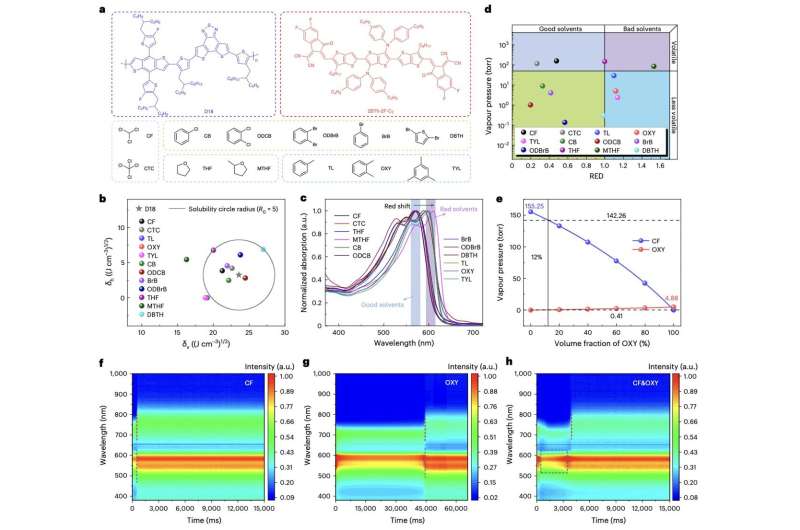
Selection of supplies and solvents. a, The chemical constructions of D18, 2BTh-2F-C2 and associated solvents. Solvents with low volatility and low solubility for the polymer donor are framed in a blue field; Solvents with excessive volatility and low solubility for the polymer donor are framed in a violet field; Solvents with low volatility and excessive solubility for the polymer donor are framed in a inexperienced field; Solvents with excessive volatility and excessive solubility for the polymer donor are framed in a bluish-purple field. b, The solubility of D18 in varied solvents within the δv–δh diagram (δh, molecular hydrogen bonding interactions; δv, δV = √δ2 D + δ2 P). c, Normalized absorption of D18 in numerous solvents. The blue rectangle represents the absorption peak of D18 in good solvents; the violet rectangle represents the absorption peak of D18 in poor solvents. d, Solvent classification diagram based mostly on vapor strain and solubility. Good solvents present a RED index smaller than 1, which is throughout the solubility sphere; Poor solvents have a RED index larger than 1, and the larger the RED quantity, the more severe the solubility. e, The vapor strain as a operate of the amount fraction of the binary solvent of CF&OXY. The strong vertical line represents the 12% OXY quantity fraction of the solvent combination, the higher dashed horizontal line represents the CF vapor strain of the 12%-OXY solvent combination for 142.26 torr, and the decrease dashed horizontal line represents the OXY vapor strain of the 12%-OXY solvent combination for 0.41 torr. If OXY takes up a lot of the solvent combination, the vapor strain of CF and OXY are each 4.88 torr with the identical evaporation charge. f–h, Time-dependent contour maps of in situ UV-vis absorption spectra for D18:2BTh-2F-C2 mix precursor options in CF situation (f), OXY situation (g) and CF&OXY situation (h). The dashed strains and dashed field symbolize the spectral change time for D18 and 2BTh-2F-C2 in CF-, OXY- and CF&OXY-based mix precursor options in the course of the movie formation course of. Source: Zeng et al. (Natural Energy2024).
As a part of their research, Zeng, Zhang and their collaborators first designed and synthesized a compound combination containing CF and OXY. They then noticed how a donor polymer and NFREA responded to this solvent combination, focusing particularly on the formation of movies of those compounds.
“We selected solvents comparable to CF and OXY that evaporate at totally different temperatures and charges and have totally different solubility for the donor polymer D18,” the researchers wrote. “Upon evaporation of chloroform, D18 begins to assemble into fibrils. Then, evaporation of o-xylene induces the fast formation of a fibril community that’s section separated 2BTh-2F-C2 into pure domains and results in a bicontinuous morphology.”
The researchers additionally recognized a solid-state additive, particularly 1,4-diiodobenzene (DIB), of their pattern. This additive is positioned on the fashioned photoactive skinny movie, whereas it’s about to dry, to additional improve the crystallization of NFREA.
The researchers used their technique to create new photo voltaic cells based mostly on NFREAs, which they evaluated in a collection of preliminary checks. Remarkably, they discovered that the morphology created by their solvent and additive enabled PCEs of 19.02% for a small space (0.052cm2) cells and 17.28% for 1 cm2 instruments.
This current research opens new potentialities for the manufacturing of natural photo voltaic cells based mostly on NFREAs, which can be cheaper than their FREAs-based counterparts. The promising findings gathered by this analysis group will quickly encourage additional efforts on this course, probably contributing to the longer term commercialization of natural photo voltaic cells.
More info:
Rui Zeng et al, Achieving 19% effectivity in non-fused ring electron acceptor photo voltaic cells by solubility management of donor and acceptor crystallization, Natural Energy (2024). DOI: 10.1038/s41560-024-01564-0
© 2024 Science X Network
Citation: A brand new method to spice up the effectivity of non-fused ring electron acceptor photo voltaic cells (2024, July 12) retrieved 12 July 2024 from https://techxplore.com/information/2024-07-approach-boost -efficiency-fused-electron.html
This doc is topic to copyright. Except for any honest dealing for the aim of personal research or analysis, no half could also be reproduced with out written permission. Content is supplied for informational functions solely.
[ad_2]
Source link