[ad_1]
A examine led by Russia’s Skoltech and China’s HPSTAR means that rubidium and cesium components can enhance the effectivity of hydrogen batteries. Researcher Dmitrii Semenok mentioned pv journal that “it’s a query of fixing the search methodology for good hydrogen storage supplies.”
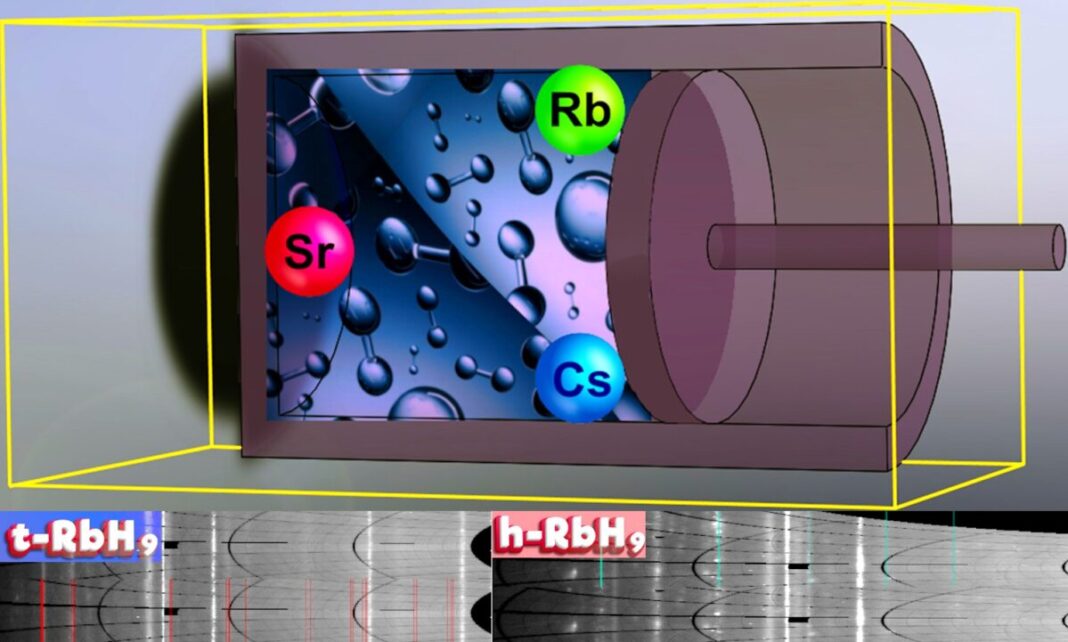
Russian, Chinese, Japanese, and Italian scientists have synthesized two polyhydrides – cesium heptahydride (CsH7) and rubidium nonahydride (RbH9) – for chemical hydrogen storage. They declare it could actually “soak up 4 instances” hydrogen used incessantly magnesium-nickel and zirconium-vanadium alloys.
“Our work reveals that cesium and rubidium have the likelihood to extend the capability of hydrogen batteries by strengthening the shell of molecular hydrogen, and opening a means for additional lowering the strain of the synthesis of polyhydride ,” written by the authors of “Raisins in a Hydrogen Pie: Ultrastable Cesium and Rubidium Polyhydride,” which was just lately printed in Advanced Energy Materials.
Cesium (Cs) and rubidium (Rs) pack seven and 9 hydrogen atoms per metallic atom, respectively.
“The proportion of hydrogen atoms in these compounds is among the highest amongst all recognized polyhydrides, twice as excessive as methane CH.4,” Dmitrii Semenok, a Chinese researcher‘s Center for High Pressure Science and Technology Advanced Research (HPSTAR), mentioned pv journal.
Semenok famous that Сs and Rb are SOMEWHAT heavy atoms.
“For rubidium hydride RbH9 the mass content material of hydrogen is about 9.6% by weight,” he mentioned. “This can also be an excellent end result. The solely downside is the necessity to use strain to synthesize these compounds.
He defined that cesium and rubidium and their hydrides current two further issues. The international manufacturing of Cs and Rb is decrease than different alkali metals, and the associated fee is increased than lithium, sodium or potassium. They are additionally extremely reactive metals that burn simply in air.
So the compounds will not be good for storing hydrogen, however the examine led by Russia‘s Skoltech and in China HPSTAR instructed that rubidium and cesium components might enhance the effectivity of present hydrogen batteries.
“It’s a query of fixing the way in which we seek for promising hydrogen storage supplies,” Semenok mentioned. “We work at a lot increased pressures than beforehand accepted on this subject. And we use components that have a tendency to provide increased polyhydrides at decrease pressures.
He added that methods like Strontium-Rubidium Sr-Rb-H, Strontium-Rb-Zirconium-H, Rb-Magnesium-H could also be promising for hydrogen storage. They stay secure below ambient situations after being saturated with hydrogen at excessive pressures of a number of GPa or much less.
“The formation of upper cesium and rubidium hydride at a comparatively low strain of about 10 GPa was predicted theoretically greater than 10 years in the past within the works of the group of E. Zurek (UB). The downside is simply the experimental validation of these outcomes,” mentioned Semenok. “Since the invention of sulfur trihydride (H3S), the principle consideration of the experiments was centered on the superconducting hydride.
The scenario has just lately modified as a consequence of fatigue within the class of binary superconducting hydride. “The enlargement of the sphere of analysis into alkali and alkaline earth metals in 2021-2022 instantly led to the invention of secure molecular polyhydrides of Cs, Rb, Ba and Sr, which was predicted again in 2012 -2015”.
In the paper, the researchers additionally proposed a brand new methodology for the synthesis of metallic polyhydride via high-pressure thermal decomposition of the corresponding amidoboranes in diamond anvil cells.
“We now intend to repeat the experiment utilizing giant hydraulic presses at a decrease strain – about 10,000 atmospheres – to acquire bigger quantities of cesium and rubidium polyhydride and show that after synthesized, it which compounds stay secure even at atmospheric strain. Unlike different polyhydrides recognized to date. This is a crucial activity from a elementary viewpoint. We have to reply the query of how lengthy the metastable polyhydride to exist below the situations of the setting, “says Semenok, including that it could actually exist for a very long time – a fraction of seconds or a whole lot of days.
This content material is protected by copyright and will not be reused. If you wish to cooperate with us and wish to reuse a few of our content material, please contact: [email protected].
[ad_2]
Source link