[ad_1]

IMAGE©Chemical Potential Analysis as an Alternative to the Van’t Hoff Method: Hypothetical Limits of Solar Thermochemical Hydrogen.
Can the Chemical Potential Analysis methodology be higher than the van’t Hoff methodology?
NREL’s Stephan Lany proposes a brand new widespread language to assist photo voltaic thermochemistry researchers select the redox supplies that can most effectively produce inexperienced hydrogen. He revealed the proposal of Journal of the American Chemical Society Chemical Potential Analysis as an Alternative to the Van’t Hoff Method: Hypothetical Limits of Solar Thermochemical Hydrogen.
Why is that this vital?
Solar thermochemical splitting of water and carbon dioxide is a promising method to convert photo voltaic power into fuels on a big scale, probably including one other renewable know-how to interchange fossil-fuel-generated hydrogen and aviation gasoline.
Ceria (CeO2) is at the moment the benchmark oxide materials for water splitting with a two-step discount and oxidation cycle. (Here, discount is the discharge of oxygen from the strong oxide, and oxidation is the replenishment of O from H2O vapor, producing H2). However, the manufacturing of hydrogen in CeO2 could be very restricted except the discount is carried out at very excessive temperatures of 1500°C or larger.
Although some photo voltaic researchers have approached the issue by designing reactors and receivers able to these larger temperatures, the seek for a brand new redox materials that may be as efficient as ceria in splitting water throughout of oxidation however the launch of extra oxygen throughout discount under 1400 ° C generally is a game- changer for these photo voltaic fuels.
“There are tens of 1000’s of oxides identified by their composition and crystal construction,” he defined in a name from Colorado. “But not all of them have been characterised and evaluated to see in the event that they work. But, how do we all know how good a brand new predicted candidate materials will likely be?”
Lany proposed a distinct methodology of study to make sure that concept and experiment are on the identical web page and create a optimistic suggestions loop, which is a requirement for the event of latest supplies or analysis. methods to enhance ceria.
How the normal methodology of study works
The conventional analytical methodology for figuring out response enthalpies and entropies for processes comparable to photo voltaic thermochemical gasoline manufacturing is the van’t Hoff methodology, which Lany describes as considerably outdated and outdated.
The limitation of this methodology is that it mixes the properties of the probe gasoline with the oxides being studied, which can obscure the statement of properties which are vital.
“In thermochemistry, what we’re , from a proper form of chemistry standpoint, is free power,” Lany defined.
“Free power is form of the governing equation and it is a normal rule of chemistry that any course of works to lower free power. It’s what drives all reactions. And if we glance at- Let’s see what the free power of the discount response is, then there’s a half that has to do with the properties of the strong state. That implies that the strong oxide, in its oxidized and in its lowered type. There can also be an element that comes from the truth that we carry oxygen from the oxide to the gasoline section. The free power has two contributions, the enthalpy and the entropy. And the whole free power is the enthalpy minus the temperature occasions the entropy. Van’t Hoff separated the free power into enthalpy and entropy, however he didn’t separate the half from the oxide in its strong state and the half from the oxygen within the gasoline section.
Why is the chemical potential evaluation methodology higher?
However, Lany took a brand new methodology, the chemical potential methodology of study, which helps to raised perceive the mechanisms of the defect and its impact on the fabric’s habits. The chemical potential methodology not solely separates the strong and gasoline section contributions, but in addition permits the evaluation of the temperature dependence of enthalpy and entropy, which may reveal vital particulars wanted to design higher supplies. .
“This is a extra trendy means of processing information utilizing extra freely identifiable relationships. The idealized linear relationship of the normal van’t Hoff methodology could be very helpful within the days when information evaluation is finished utilizing graph paper and a ruler, however it solely holds fixed enthalpy and entropy. With the chemical potential methodology, we are able to simply decide the dependencies on temperature,” he stated.
This new methodology permits a extra detailed evaluation of the habits of supplies, which gives vital clues in regards to the chemical and bodily mechanisms that decide the thermochemical properties.
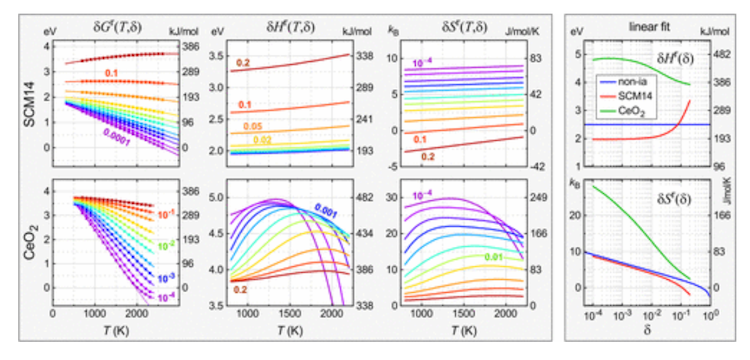
Lany’s examine examined this new methodology of study in three computational mannequin techniques together with ceria, which displays distinctive habits because of the means electrons can redistribute when the O atoms are faraway from the crystal lattice. This course of is temperature dependent and finally accountable for CeO2’s capacity to type concentrated H2/H2O mixtures whereas most different oxides dissociate into water, if in any respect, then underneath very dilute circumstances. like 1/1000.
Tracing the temperature dependence of enthalpy and entropy is one method to determine the habits that makes CeO2 so particular. Lany’s hope is that it will turn out to be a brand new widespread language to make the connection between experimental observations and theoretical fashions. He stated: “I feel this can be a very helpful concept for folks in thermochemistry who do thermogravimetric evaluation and attempt to extract insights in regards to the mechanism of discount of supplies from their measurements.”
A balancing act
Although altering ceria or discovering new oxides can enhance efficiency, good points within the discount step usually worsen the oxidation step in redox cycles, limiting hydrogen manufacturing. The objective is to steadiness the discount and oxidation properties to optimize the efficiency of the fabric for photo voltaic gasoline technology.
“I take ceria as a baseline materials, after which I say, Okay, what sort of properties does a distinct materials want to enhance the habits of ceria,” he stated.
“I’m simply questioning if we are able to design supplies which are related however completely different from ceria – what can we do for water splitting supplies? I’m all the time on the aspect of latest supplies, however I would not rule out risk of modifying ceria; which is normally one thing like alloying or doping the fabric, the place we add a number of further parts.”
Lany’s paper describes how the proposed new widespread language is used to attach completely different defects and materials properties to thermochemical properties (temperature dependence of enthalpy and entropy) and from there to water splitting. carried out on current and hypothetical supplies, particularly each the whole H2 output and the H2/H2O ratio of the oxidation step.
Further studying: Chemical Potential Analysis as an Alternative to the Van’t Hoff Method: Hypothetical Limits of Solar Thermochemical Hydrogen
3D-printed ceria is a game-changer to extend photo voltaic gasoline effectivity
How does Synhelion’s photo voltaic receiver obtain such excessive temperatures?
Published in Materials Today Energy – Strategic co-doping of ceria for improved oxidation kinetics in photo voltaic thermochemical gasoline manufacturing
[ad_2]
Source link