[ad_1]
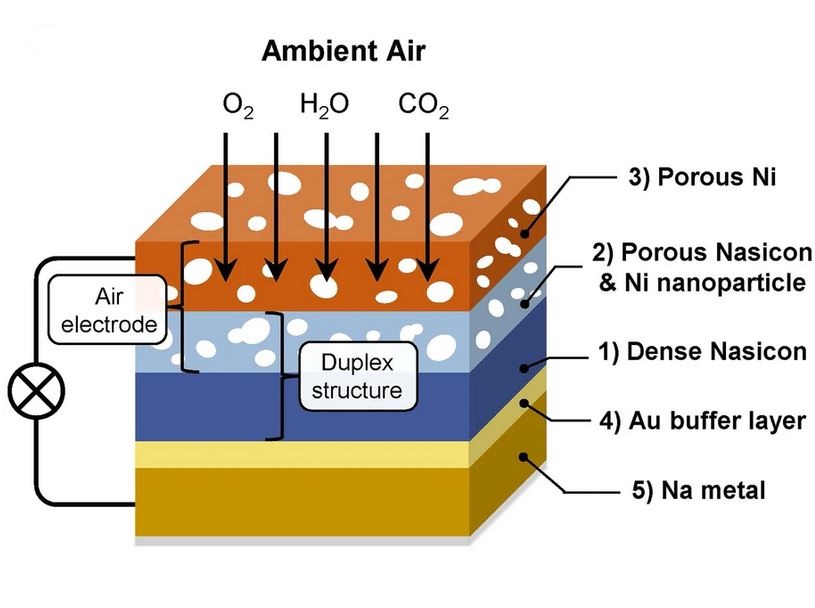
Schematic of the battery
Image: Pohang University of Science and Technology (POSTECH), environmental communication, Common License CC BY 4.0
From pv journal ESS News web site
Metal-air batteries, which use lithium or sodium, are of nice curiosity due to their exceptionally excessive theoretical gravimetric power densities. These batteries rely largely on the usage of pure oxygen for the formation and decomposition of steel oxides, relatively than ambient air.
While ambient air is a extra sensible choice, CO2 and H2O within the air may cause extreme irreversible reactions, such because the formation of carbonates and hydroxides, which regularly injury the battery. To handle this, metal-air batteries normally require extra gear, resembling an oxygen permeation membrane to purify the oxygen or selectively use atmospheric oxygen.
Now, researchers from Pohang University of Science and Technology (POSTECH) in South Korea have developed a high-energy, high-efficiency all-solid-state sodium-air battery that may recycle sodium and air with out would require particular gear.
The group used Nasicon, a sodium superionic conductor and stable electrolyte, to resolve the carbonate challenge. Nasicon, which accommodates components resembling sodium, silicon, and zirconium, permits ion motion within the stable state whereas exhibiting excessive electrochemical and chemical stability.
Using this stable electrolyte, the group protected the sodium steel electrodes from air and accelerated the breakdown of carbonate shaped throughout electrochemical cell operation.
To proceed studying, please go to our ESS information web site.
This content material is protected by copyright and might not be reused. If you wish to cooperate with us and wish to reuse a few of our content material, please contact: editors@pv-magazine.com.
[ad_2]
Source link